 Chiu Laboratory
Chiu Laboratory
Cryo-EM of Membrane Proteins
May 07, 2022.
Our research focuses on biomolecular mechanisms, particularly membrane receptor complexes and energy-related machinery in biological systems. We visualize these molecular processes using electron cryogenic microscopy (cryo-EM), and the main approaches we use are electron crystallography (two-dimensional (2D) crystal specimens or helical objects), electron tomography, and single-particle cryo-EM. We are also exploring new imaging and sample preparation methods to push the limits of cryo-EM use in biology.
Membrane protein acts as a gate keeper or a diode in cell membranes, which plays an important role in maintaining physiological processes in response to environmental changes. We are tackling the fundamental questions about:
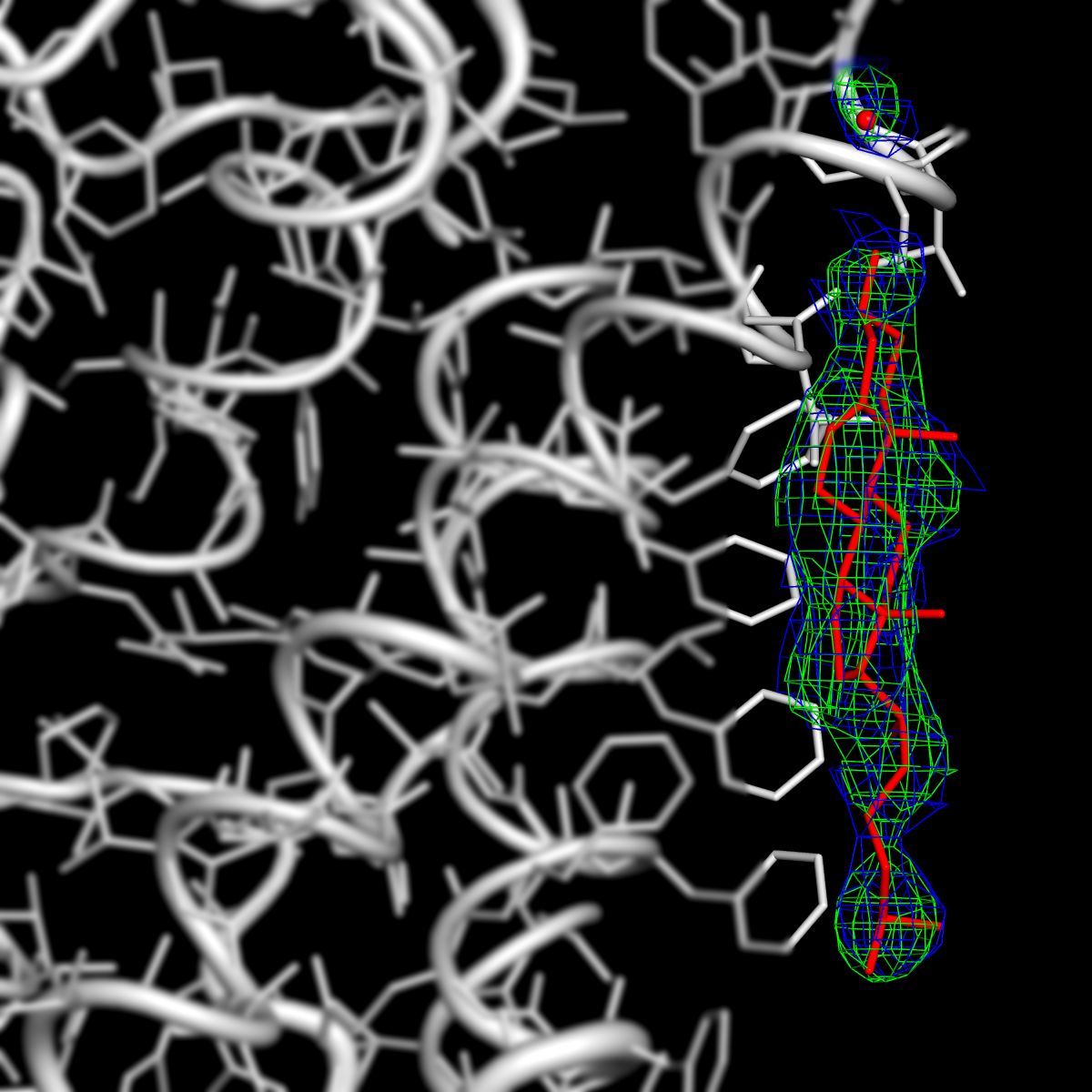
 Aquaporin-0 (AQP0) with membrane lipids.
Aquaporin-0 (AQP0) with membrane lipids.

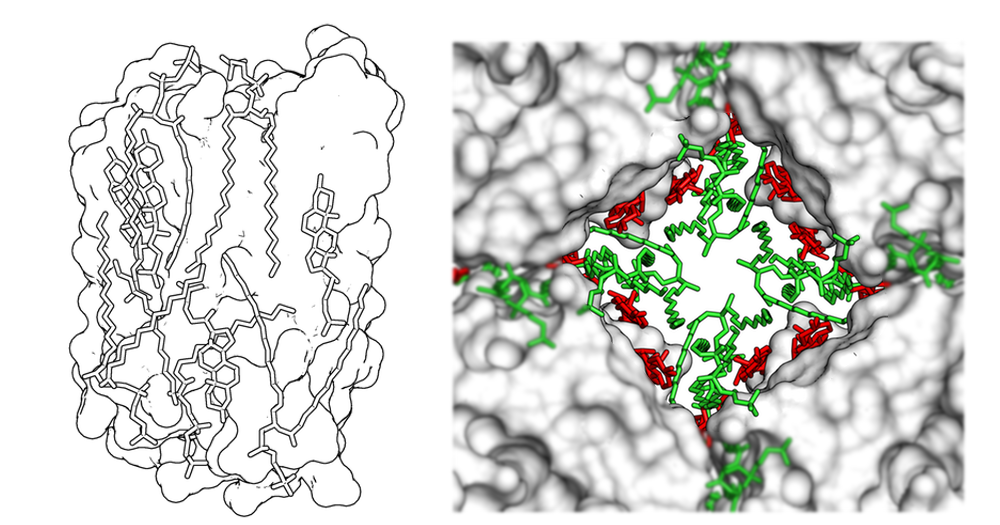
 Negatively-stained MloK1 revealed by electron crystallography and single-particle reconstruction.
Negatively-stained MloK1 revealed by electron crystallography and single-particle reconstruction.
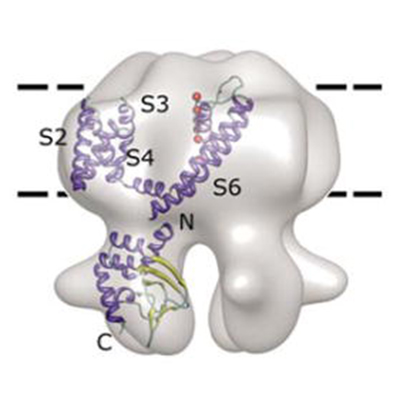
 Schematic of the receptor complex of pro-NGF-p75NTR-sortilin. p75NTR functions as a switch of the fate of a neuron, depending on binding co-receptors. Pro-NGF is the precursor of the nerve growth factor, which binds p75NTR and sortilin to initiate neuronal apoptosis. The pro-domain plays an important role in determining the apoptotic pathway. We are interested in the molecular underpinnings of how pro-domain engages in the receptor complex formation and leads to neuronal apoptosis.
Schematic of the receptor complex of pro-NGF-p75NTR-sortilin. p75NTR functions as a switch of the fate of a neuron, depending on binding co-receptors. Pro-NGF is the precursor of the nerve growth factor, which binds p75NTR and sortilin to initiate neuronal apoptosis. The pro-domain plays an important role in determining the apoptotic pathway. We are interested in the molecular underpinnings of how pro-domain engages in the receptor complex formation and leads to neuronal apoptosis.
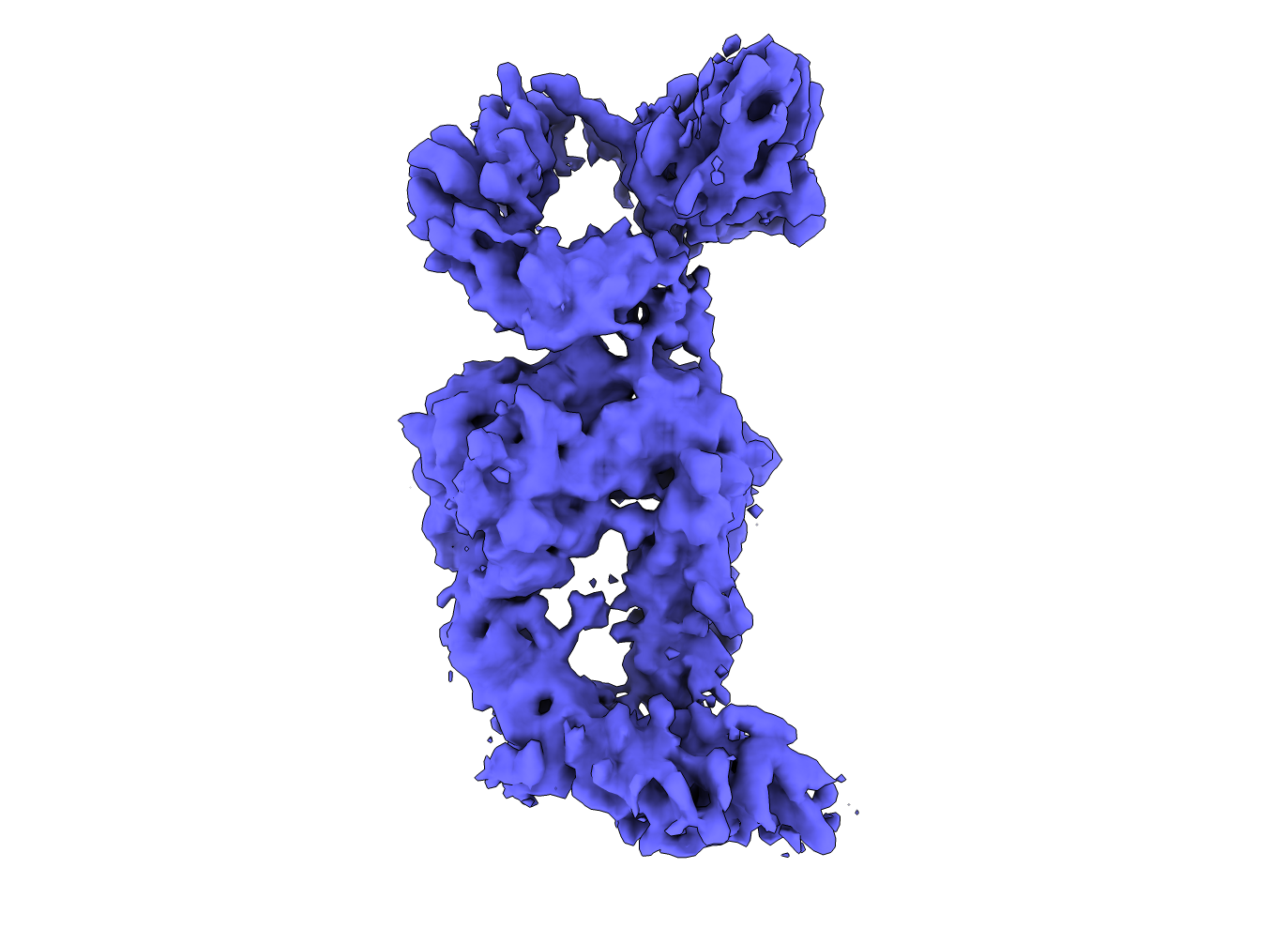 Cryo-EM density of an integrin complex.
Cryo-EM density of an integrin complex.
We are interested in how a cell produces or utilizes energy to sustain its life and how different organisms evolve these molecular machinaries to adapt to the environmental changes. We are currently investigating: (1) regulatory mechanism of the ATP synthase nanomotor for ATP generation; and (2) how a photosynthetic bacteria maintains high energy transfer efficiency in an extremely low-light condition.
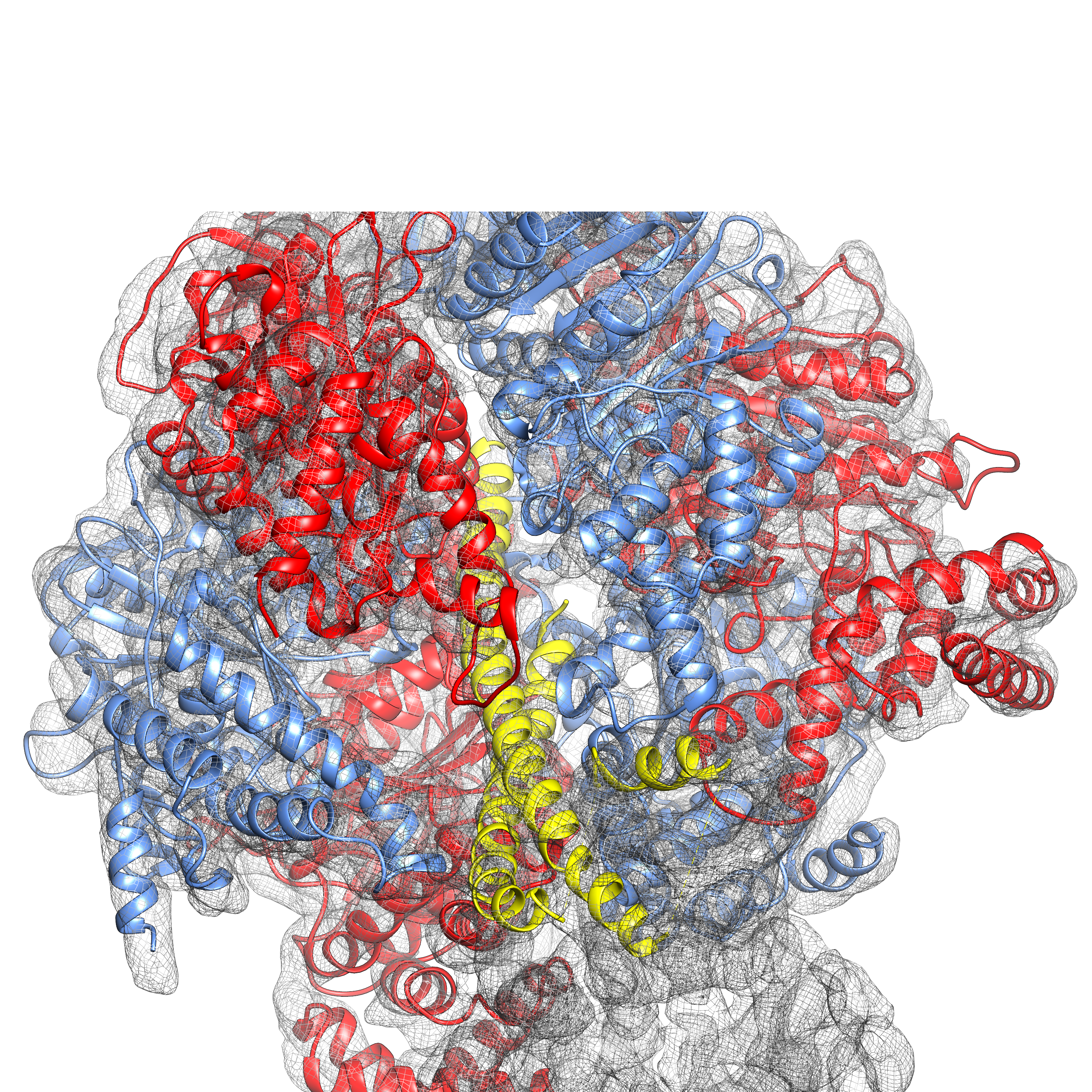
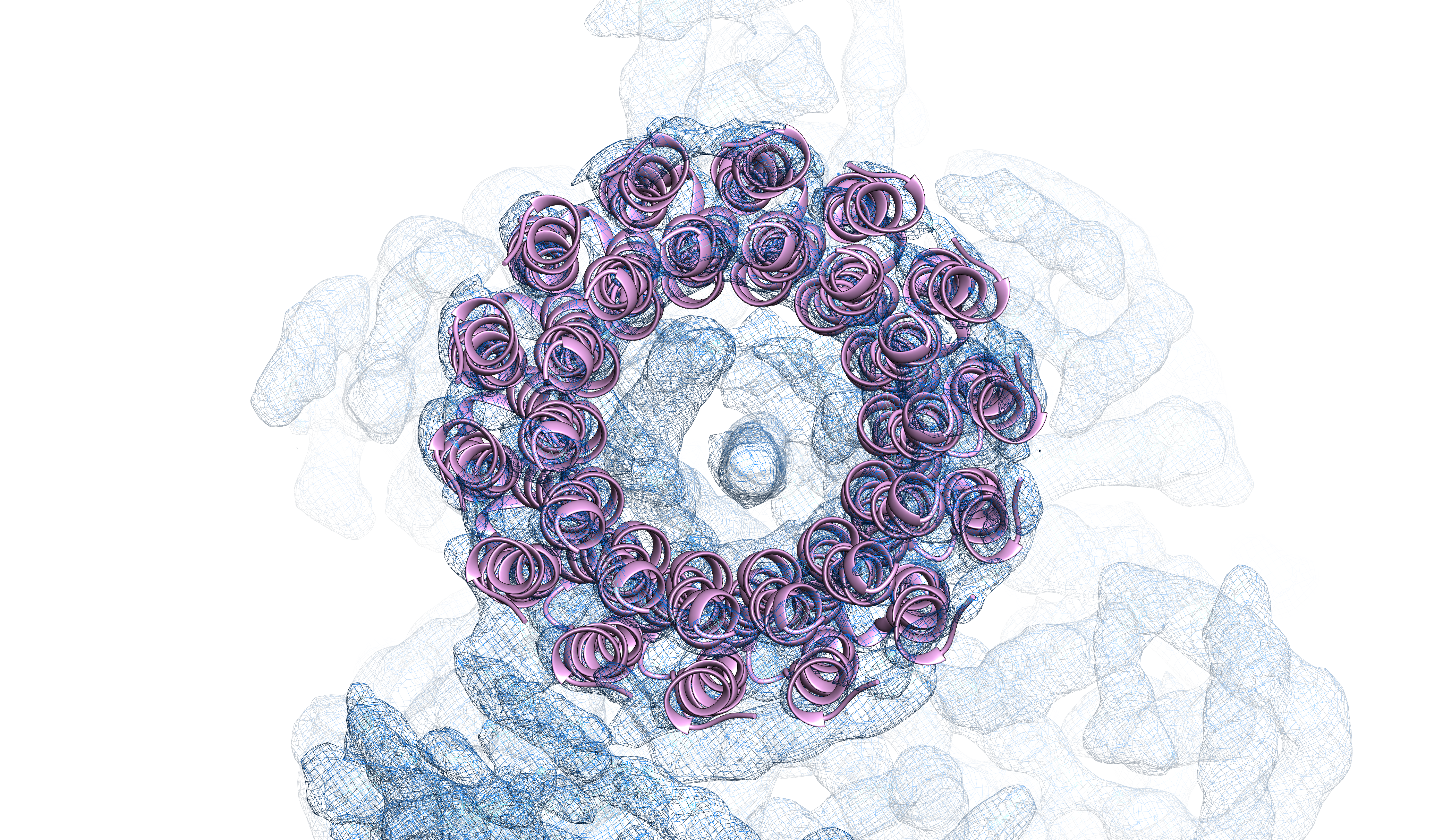 Single-particle cryo-EM structure of an F-type ATP synthase (Left: F1 density; Right: transmembrane c-ring).
Single-particle cryo-EM structure of an F-type ATP synthase (Left: F1 density; Right: transmembrane c-ring).
We are exploring electron holographic imaging for the use in the measurements of single-particle physical properties. We initially focus on the specimen of a polarized membrane.
Arizona State University (ASU) has a long history of international leadership in the field of electron microscopy. The Center for High Resolution Electron Microscopy, established as a National Science Foundation (NSF) Regional Center by Professor John M. Cowley in 1980, currently houses three aberration-corrected transmission electron microscopes (TEMs) and one Titan Krios TEM. The existing resources for structural biology studies on the campus include the Magnetic Resonance Research Center (MRRC), the College of Liberal Arts and Sciences (CLAS) Electron Paramagnetic Resonance (EPR), and the CLAS-EM Facility. Additionally, ASU has pioneering research groups in the field of X-ray free electron lasers (XFEL) and femtosecond nano-crystallography. The Center for Applied Structural Discovery (CASD) at the Biodesign Institute has the world’s first high energy Compact Free Electron Laser (CXFEL) hosted in the basement of the Biodesign C building, providing new researhc opportunities for scientists who study structural biology and chemistry.